
“I say yes to participating in research because of my grandchildren. If this clinical trial doesn’t help me, it may help one of them.”
– Dale Adlem, Sechelt
Every month, Sechelt resident Dale Adlem makes the trip into Vancouver to participate in a clinical trial for neovascular age-related macular degeneration (or wet AMD)–a chronic eye disease she was diagnosed with last year that is the most common cause of permanent and irreversible vision loss in people over 65. The trip is not the easiest outing for the 83-year old, with a ferry ride and traffic to get through, but the thought of her grandchildren and the possible impact that this study may have on their lives motivates her to keep going.
“When I was first asked if I’d be interested in joining the study, I thought, ‘Oh, I don’t know’,” says Adlem. “Then I thought about my grandchildren–I have two girls coming up–and if this research doesn’t help me, it may help one of them.”
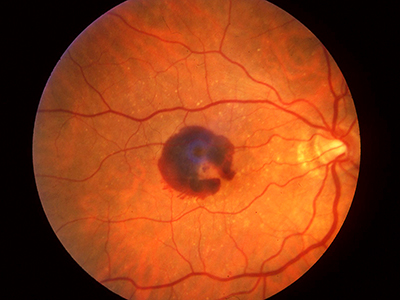
Wet AMD occurs when new blood vessels start to grow underneath the retina, mainly as a result of aging changes in the eye. These new blood vessels in the back of the eye lead to bleeding and scarring in the macula, i.e. the centre of the retina, and the damage to the macula results in rapid vision loss. Treatment for wet AMD has been revolutionized over the past five years thanks to new drugs that target vascular endothelial growth factor (VEGF), a molecule that mediates the growth and leakage of abnormal blood vessels in this disease. Anti-VEGFs are the first drugs to help wet AMD patients actually regain some vision, rather than simply slowing the progression of blindness, which is what previous treatments did.
While anti-VEGFs have helped patients regain vision, recent research shows that the medications need to be administered regularly for the rest of the patient’s life, which is a treatment burden for both patients and doctors. Also, recent studies indicate that after a few years of using anti-VEGFs to treat wet AMD, patients start losing vision again but a slower rate than prior to being treated. Eventually, patients on anti-VEGFs still lose vision because of progression of atrophy and scarring.

“The hope with something like this is that we’ll get better visual acuity results in the long term, less progression of atrophy, and decrease the treatment burden,” says Dr. Forooghian.
“Anti-VEGFs don’t cure wet AMD, they just provide a temporary treatment,” says Vancouver Coastal Health Research Institute (VCHRI) clinician-investigator Dr. Farzin Forooghian, director of research for the Vancouver Retina Research Group (VRRG) at the Eye Care Centre (VGH) and St. Paul’s Hospital. “Anti-VEGF drugs are not enough, and we need other molecular targets in order to better treat this disease.”
Dr. Forooghian, who is also a clinical assistant professor in the Department of Ophthalmology & Visual Sciences at the University of British Columbia (UBC), is principal investigator for A Phase 3 Safety and Efficacy Study of Fovista™ Intravitreous Administration in Combination With Lucentis® Compared to Lucentis® Monotherapy, also known as the Fovista study. Fovista is a new drug that targets platelet-derived growth factor (PDGF). By blocking PDGF, new blood vessels causing wet AMD die off. Preliminary data have suggested that adding Fovista (anti-PDGF) to anti-VEGF treatment could potentially lead to better vision, less scarring, and less treatments overall.

Focus on differeent target advances evolution of wet AMD treatment
“This is a pivotal clinical trial. Over the years, treatment of AMD has progressed from using thermal laser and surgery, to photodynamic therapy combined with pharmaceuticals, and then to the anti-VEGF pharmaceuticals that came from the area of anti-cancer research,” says Dr. Patrick Ma, VCHRI researcher and principal investigator for the Fovista study at VGH. “This clinical trial offers patients a new approach that was not previously available to them.”
“We have conducted similar trials, which have shown benefits, and have come a long way since the first wet AMD treatments,” he adds. “However, this is a new pharmaceutical drug class being tested and I am hoping it will bring improved benefits to our patients.”
For their participation, study participants must visit the research team once per month, get an eye exam, and an injection. The clinical trial sponsor pays for the medications administered and patients are compensated for their travel.
For her part, Adlem will continue to make the monthly journey to participate in the clinical trial, even though she would rather be tending to her big garden on the Sunshine Coast.
“I would think anybody who has the health to participate in these kinds of studies and can do it, should do it, regardless of age,” she says. “Okay, it’s an inconvenience for me to get on the 6:20 ferry in the morning, get into town, get a driver if I need one, and fight traffic, but yes, I’d still definitely suggest it to anyone to participate.”
THIS IS ONE PATIENT’S STORY OF PARTICIPATING IN A CLINICAL TRIAL. YOUR EXPERIENCE MAY DIFFER. LEARN MORE ABOUT CLINICAL TRIALS BEFORE PARTICIPATING.


