
Implanted technologies supported by this grant could dramatically improve treatment outcomes for paralyzed patients.
A $48 million (USD$36.4) grant has been awarded to researchers at Vancouver Coastal Health Research Institute (VCHRI) and the University of British Columbia (UBC) to investigate integrative and implantable technology that could revolutionize how patients recover from traumatic spinal cord injury (SCI).
“We are extremely excited to embark on this journey,” says team lead and VCHRI researcher Dr. Brian Kwon. “Never before has an agency committed funding of this magnitude for spinal cord injury and set such a high bar for not just incremental, but truly transformative solutions.”

The five-year grant awarded by the Defense Advanced Research Projects Agency (DARPA)—a US-based agency known for funding the development of novel high-risk, high reward technologies—brings together a multi-disciplinary team to develop a revolutionary treatment for “bridging the gap” in an injured spinal cord.
The internationally renown team of collaborators includes Dr. Karen Moxon from the University of California, Davis; Dr. Mark Tuszynski at the University of California, San Diego; and Dr. Gregoire Courtine at École Polytechnique Fédérale de Lausanne in Switzerland.
“We expect that this technology will enable us to personalize and optimize treatment, particularly during the early stages of injury,” says Kwon.
“This approach could lead to improved movement, including better functional control over the arms and hands, and enhanced walking ability.”
A multipronged approach to improve function after a spinal cord injury
Starting with the early injury phase, Kwon’s protocol involves implanting a near-infrared spectroscopy (NIRS) device into a patient’s cervical spine to monitor blood flow, pressure and oxygenation levels. Vital signs from the NIRS device, which emits non-tissue-damaging invisible light, would be displayed on a computer or tablet through a wireless connection—and could one day be connected to wearable technology or a smartphone.
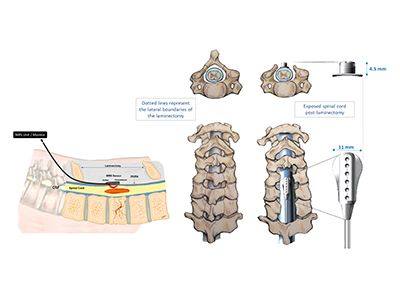
Paired with this NIRS device is a targeted electrical spinal stimulation device for hemodynamic control (TESS-HEMO). The TESS-HEMO’s customized electrode array is surgically implanted around the lower part of a patient’s spinal cord. It delivers precisely timed bursts of electricity to specific neurons that influence and adjust blood pressure to ensure that the spinal cord receives enough blood and oxygen.
“Currently, clinicians often need to make a ‘best-guess’ about how well blood is circulating through the injury site, and then attempt to make adjustments accordingly,” says Kwon. “The NIRS monitoring system paired with TESS-HEMO would give clinicians real-time information, enabling them to more precisely optimize the injured spinal cord’s blood supply to improve outcomes.”

Reduced blood flow can result in preventable neurological damage. By being in a better position to stabilize and monitor patients’ blood flow post-surgery, Kwon anticipates that acute patients will experience a speedier recovery with the potential for greater control over their extremities, as well as better bladder and cardiovascular outcomes.
Applying this approach to long-term SCI patients could address chronic conditions, such as severe hypotension—low blood pressure—which can cause patients to faint when moved from a prostrate to a sitting position, or even from a slightly reclined to a slightly more upright position. With time, chronically reduced blood pressure can starve the brain of oxygen, eventually leading to long-term brain damage, says Kwon.
A third technology the team will develop is neuronal stem cells, which will be transplanted into the damaged spinal cord within personalized, 3D-printed biodegradable scaffolds. This combination will attempt to reestablish communication and control over such things as heart, bladder and motor function.
“Even a small gain in motor function, such as being able to grasp a pen again, can be quite life-changing for an individual with a spine injury,” says Kwon.
“This would be the first in-human transplantation of the scaffold into a patient with a cervical SCI.”
Much of the work funded by the DARPA grant will be centred at UBC and VCHRI, which Kwon notes is a credit to the specialized multidisciplinary care for acute SCI at VGH and his team of scientists, technicians and trainees at his ICORD-based lab. Key UBC collaborators on the project include Dr. Babak Shadgan of the Department of Orthopaedics and Dr. Chris West of the Department of Cellular and Physiological Sciences.
“Without them and their collective passion for advancing research and care for spinal cord injuries, none of this would be possible.”


