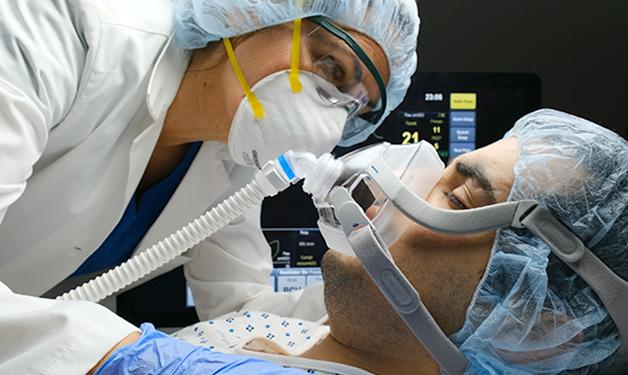
Efforts that include pre-clinical research at the Jack Bell Research Centre could provide essential care for vulnerable patients.
A non-invasive device designed to improve outcomes for patients on mechanical ventilators was recently authorized by the FDA for emergency use during the COVID-19 pandemic. The Lungpacer Diaphragm Pacing Therapy System (DPTS) was developed to assist with retaining and regaining diaphragm function that, once lost, can make it challenging for patients to be taken off a ventilator.
“Mechanical ventilation is a potentially life-saving intervention, but there are still real and significant challenges that require innovative solutions to improve patient outcomes,” says Matt Gani, vice president of research with Lungpacer Medical Inc (LMI).

Mechanical ventilation moves air into the lungs to oxygenate the blood and remove carbon dioxide. The procedure is administered to patients who are experiencing severe difficulty breathing due to such things as inflammation and fluid build-up in the lungs, along with patients undergoing surgery and with other conditions.
Almost 42 per cent of COVID-19 patients develop acute respiratory distress syndrome1, which deprives the lungs of oxygen and can require mechanical ventilation. On average, around 33 per cent of all intensive care unit patients in Canada will require mechanical ventilation at some point during their stay2.
Findings from an Ontario research study forecast a 57 per cent increase in ventilator use by 2026 based on 2006 usage3.
While the use of ventilators is a necessary life-saving intervention, it can negatively impact some systems in the body, such as the diaphragm.
Ventilator-induced diaphragmatic dysfunction (VIDD) occurs when the diaphragm is relieved of its duties due to mechanical ventilation, causing the diaphragm to atrophy and shrink.
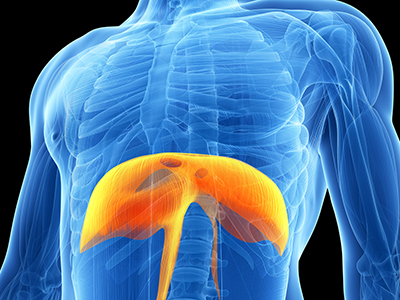
VIDD has been shown to manifest within 18-69 hours following treatment administration, and can weaken the diaphragm muscle to a point where taking a patient off a ventilator becomes difficult.
Lungpacer DPTS is expected to slow or prevent the diaphragm from shrinking, or reverse diaphragm atrophy, by sending electrical signals through the phrenic nerves in the patient’s neck and chest to activate the diaphragm muscle.
This video, produced by Lungpacer Medical Inc., explains the clinical application of Lungpacer technology:
Researchers hypothesize that Lungpacer DPTS may not only make it easier for patients to be weaned off a ventilator and breathe on their own again, it may also shorten the clinical course of ventilator use, says Gani.
While not yet FDA-approved, as of April 2020 the Lungpacer DPTS received Emergency Use Authorization by the FDA to be administered for up to 30 days to assist with weaning patients 18 years of age and older off mechanical ventilation if they are at high risk of weaning failure.
More research is needed to discover the Lungpacer’s full potential
The research technicians at the Jack Bell Research Centre (JBRC) have been working closely with the LMI team to develop the Lungpacer DPTS technology since 2009.
JBRC’s highly trained technicians collaborate with and support many groups with pre-clinical research by providing laboratory-based research support, space, materials, supplies and technical assistance, along with administrative guidance on such things as ethics approvals.
“We were fortunate to have been able to conduct sufficient investigative work with LMI prior to the outbreak of COVID-19 to see the Lungpacer therapy put to work during this pandemic,” says assistant manager with JBRC, Kate Orchard. “We hope this technology can be used to help a lot of people.”

In early 2020, LMI pivoted its operations to include the emergency clinical application of Lungpacer DPTS. At the same time, Gani says LMI’s sights continue to be set on exploring the Lungpacer’s full potential for all patients in need of mechanical ventilation, which could encompass surgical and long-term care patients.
“The studies we are conducting right now will help us evaluate the optimal ways the Lungpacer therapy can be utilized.”
“Our longstanding relationship with the Jack Bell Research Centre has enabled us to conduct some very important studies to investigate this therapy and generate information, data and scientific findings that hopefully highlight the potential benefits of Lungpacer technology to the world at large.”
1 Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China
2, 3 Canadian Institute for Health Information - Care in Canadian ICUs


