Personalized, precision therapies are more effective and efficient for prostate cancer patients.
Movember is in full swing, changing the lives of men with prostate cancer by raising both awareness of the disease and funds for research. One program made possible by these funds is My Precision Oncology Program (MyPOP), recently launched by the Vancouver Prostate Centre (VPC).
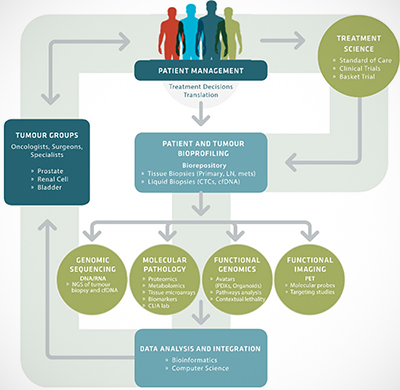
MyPOP aims to tailor treatments to patients by linking their genomic (DNA) sequencing data with clinical decision making and by integrating clinical information with novel model systems.
“What makes the VPC’s approach different is that all of us here have a deep understanding of the unique genomics and biology of aggressive prostate cancer, which enables us to focus on genes and mechanisms that are specific to these men,” says Dr. Alexander Wyatt, senior research scientist at the VPC, a national Centre of Excellence for commercialization and research hosted by Vancouver Coastal Health Research Institute and the University of British Columbia (UBC).

Through MyPOP, VPC and BC Cancer Agency patients with advanced or treatment-resistant cancer of the prostate, kidney, or bladder, can access treatment science such as clinical trials and studies. MyPOP incorporates bioprofiling of patients via tissue biopsies or by a novel technique called liquid biopsy, which is being developed at the VPC. The liquid biopsy approach works on the premise that circulating tumour DNA – DNA from cancer cells – floats freely in a cancer patient’s blood and therefore can be obtained via a simple blood test rather than traditional biopsy. Genomic sequencing, molecular pathology, functional genomics (including the use of avatar models), and/or functional imaging of cells and tissues from both liquid and traditional biopsies help researchers gain insights about what treatments may work best for an individual patient.
In Dr. Wyatt’s VPC lab, blood samples are processed to extract the DNA that comes from the tumour. The tumour DNA is then subjected to a barrage of genomic profiling techniques, including next generation sequencing. The process allows the researchers to determine which gene mutations are present. At the same time, Dr. Wyatt and his colleagues also look at the patient’s normal DNA inherited from their parents. By looking at the differences between the tumour DNA and the normal DNA, the researchers are able to understand what has arisen in the cancer and it also allows them to look at pre-disposing contributory factors to cancer development.
“Then we come together as a group – the VPC’s clinicians and scientists – and we discuss results together in terms of each patient’s clinical history and genomic findings,” says Dr. Wyatt, who is also an assistant professor in the Department of Urologic Sciences at UBC.

“For example, let’s say that I have a new targeted treatment that I expect to work in a specific situation that only occurs in five per cent of patients. If we test that treatment in an unselected 100 patients, that means 95 per cent of them won’t benefit at all, and we may then consider that drug ineffective even though five patients had a great response.
“We need to be selecting those five people beforehand that have the best chance of response and preferentially test the treatment in them. Because the risk is that we’re throwing away a lot of drugs that may be very effective, but only in a small proportion of patients.”
A program like MyPOP is part of the VPC’s efforts to improve patient outcomes as much as possible.
“If you can tailor therapies appropriately you can extend life,” Dr. Wyatt says. “But there’s a second outcome: We still know very little about this disease, and so every patient that we’re able to look at in this comprehensive way allows us to learn more and more. It’s not just about what drugs are available today but it’s learning about what we should be thinking about tomorrow.”
“We have to be aware that we’re really just scratching the surface of the complexity of the disease. The more patients we can look at the more patterns we can see.”