Researchers have discovered a way to destroy mycobacteria superbugs using a nano-sized approach.
Antimicrobial resistance is one of the top 10 global health threats, according to the World Health Organization. Taking the form of bacteria and fungi unresponsive to antibiotics and other treatments, these superbugs affect one in 16 Canadians admitted to hospital.
One such infection is caused by members of the mycobacteria family: a prevalent, treatment-resistant bacteria whose irregularly shaped microscopic rods spread through airborne particles of infected soil, fluids or dust. Mycobacteria targets macrophages in the body, specialist cells that form part of the immune system and are designed to detect and destroy pathogens, such as bacteria.
A new research study led by Vancouver Coastal Health Research Institute researcher Dr. Horacio Bach has identified a novel approach to destroying antibiotic-resistant nontuberculous mycobacteria.
“This is the first paper to show that you can kill multiplying intracellular Mycobacterium using an antibacterial agent instead of antibiotics.”
Bach and his research team encapsulated antibacterial agents and lignin-silver nanoparticles inside vesicles termed niosomes. This helps to internalize the nanoparticles inside macrophages. Upon binding to the cell membrane of macrophages, the niosome delivers its nanoparticle payload into the infected macrophages with detrimental consequences to the intracellular mycobacteria.
Published in the journal Nanomaterials, Bach’s study paves the way for new treatments for conditions that are particularly susceptible to Mycobacterium abscessus, a type of nontuberculous mycobacterial infection.
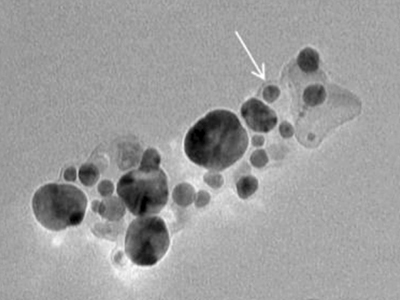
At 126.1 nanometres in size — 80,000 to 100,000 times smaller than the thickness of a human hair — the nanoniosomes Bach and his team developed were also significantly smaller than those used in other studies that investigated niosome treatments for mycobacterial infection. These typically ranged between 4.2 and 8.7 micrometres — around 70 times smaller than the diameter of a human hair.
Mycobacteria are around 0.3-0.5 micrometres, making Bach’s nanoniosomes a fraction of the size of their target.
A personalized approach could help overcome antimicrobial resistance
Responsible for tuberculosis and leprosy, along with other conditions involving the infection of macrophages, the waxy and complex cell walls of mycobacteria protect them or delay the penetration of antibiotics, such as isoniazid. Some strains can be treated with the antibiotics clarithromycin and rifamycin, but many treatment-resistant strains evade even these.
“Our arsenal of effective antibiotics is shrinking, which is why we need to find new treatment approaches to overcome this barrier.”
People with cystic fibrosis — a genetic condition that damages the lungs and other organs — along with the lung-capacity-reducing chronic obstructive pulmonary disease are more vulnerable to a severe mycobacterial infection.
“Cystic fibrosis patients cannot clear fluids from their lungs properly. This makes their lungs prone to infections and more likely to contract a strain of potentially fatal treatment-resistant mycobacterial,” says Bach.

While more research is needed to investigate the breadth of possibilities of Bach’s discovery, he believes these results are a positive indication of the future applications of the nanoniosomes he and his team have developed.
“This has the potential to become a targeted therapy for people with conditions such as cystic fibrosis, along with tuberculosis and other conditions that involve infection of the macrophages.”


