
A novel therapy shows signs that it could provide relief of major depression among patients with Parkinson’s disease.
A novel technique used to treat depression has shown promise as a means of helping relieve some of the symptoms of Parkinson’s disease, according to the preliminary findings of the ongoing Magnetic Seizure Therapy (MST) for Parkinson’s Disease clinical trial.
“Parkinson’s affects parts of the frontal cortex that modulate mood and energy levels, among other areas of the autonomic nervous system,” explains study lead, Vancouver Coastal Health Research Institute researcher Dr. Fidel Vila-Rodriguez.
“The brain is like a road, in that an accident in one area can lead to downstream impacts in other areas.”
While Parkinson’s disease initially affects dopamine cells in the brain stem that lead to motor symptoms, over time several other parts of the brain are impacted, including areas that influence mood, anxiety or energy. A progressive brain disorder, symptoms often worsen with time.
Many symptoms of Parkinson’s mirror those of depression, including apathy, sleep difficulties, decreased motor activity, a lack of enjoyment in activities and a sad demeanour visible in facial expressions.

“It is hard to disentangle whether a patient’s depression symptoms result from dopamine deficits in Parkinson’s disease or other reasons,” explains Vila-Rodriguez. “However, we know that dopamine uptake medication is often not an effective treatment for depressive symptoms.”
Magnetic seizure therapy had mood-boosting effects on one study participant
Vila-Rodriguez and his research team theorize that MST technology could help ameliorate several of the symptoms of Parkinson’s disease, including anxiety and depressive symptoms.
The MST procedure involves applying coil-shaped, electrically charged magnets to the forehead area of a patient to target the frontal cortex of their brain. The procedure triggers a controlled seizure that lasts less than one minute.
“This focal seizure becomes generalized and spreads to the rest of the brain, which we believe may regulate not only the dopamine circuits that are primarily responsible for motor symptoms but also other areas that regulate mood symptoms,” says Dr. Fidel Vila-Rodriguez.
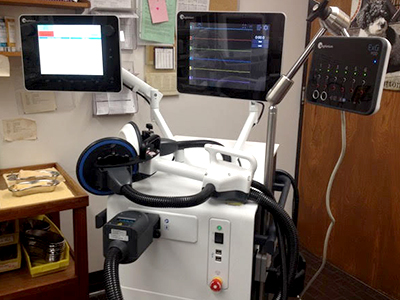
Participants in Vila-Rodriguez’s clinical trial receive treatment twice per week for up to 16 treatments. To be eligible, participants must be 50 years of age or older, and have a diagnosis of both Parkinson’s and depression.

Before participating in the clinical trial, one study participant experienced prolonged major depression that involved withdrawal from his family and a disinterest in seeing his grandchildren, both of which he had a prior fondness.
“The patient shared that he is now getting out more and enjoying activities again,” explains Vila-Rodriguez. “He is smiling, cracking jokes and spending more time with his grandkids and other family members.”
“After receiving the therapy, the patient reported feeling decreased levels of anxiety and a greater sense of relaxation.”
He also had previous difficulties getting up to use the washroom at night, dressing and feeling comfortable leaving his home to attend appointments, often requiring assistance and reassurance from his wife. Following the MST procedure, all three are now manageable enterprises.
“This result was noticeable and tangible to both the patient and his wife,” adds Vila-Rodriguez. “It has substantially improved their quality of life with no real side effects to speak of.”
The Magnetic Seizure Therapy for Parkinson’s Disease clinical trial is currently recruiting patient participants. To learn more, contact study coordinator Michelle Avina at 604-827-1361 or ninet.lab@ubc.ca.


