
Cutting-edge imaging technique could result in earlier interventions and more resources for those most in need.
One of the most prevalent and concerning complications diabetes patients face is diabetic retinopathy (DR), which can lead to partial or complete vision loss. Now, a clinical trial based out of Vancouver Coastal Health Research Institute (VCHRI) could uncover clues about how the disease progresses, enabling clinicians to intervene quickly and only when necessary.
“This research could lead to new non-invasive, non-harmful screening procedures that would tell us very early on which patients are at risk of developing severe sight-threatening diabetic retinopathy,” says Dr. Sonja Karst, a visiting scientist at the Vancouver General Hospital (VGH) Eye Care Centre.
“Being able to differentiate between patients at high- and low-risk of developing sight-threatening DR would have huge advantages for patients and clinicians.”
Diabetes is a fairly common chronic disease where the body is either unable to produce insulin (Type 1) or properly use the insulin it produces (Type 2). It causes chronic high blood sugar levels, which can damage blood vessels, nerves and organs.
Within the first 20 years of the disease, DR affects almost all patients with Type 1 Diabetes and more than 60 per cent of patients with Type 2 Diabetes. This micro-vascular complication causes damage to the small blood vessels in the retina, the light-sensitive tissue located at the back of the eye.

In its early stages, DR might not cause any symptoms. However, advanced DR can lead to fluid accumulation or to the growth of abnormal blood vessels in the eye, which can cause blurred vision, dark spots and floaters. Abnormal blood vessels can develop and lead to vitreous hemorrhage, retinal detachment and sudden vision loss.
The risk of vision loss from DR-related eye diseases makes proper diagnosis and early treatment essential.
The disease is more likely to progress to vision-threatening levels the longer someone lives with diabetes and when a patient’s blood sugar is not adequately controlled. The present challenge clinicians face is that it is still unclear who will and will not develop sight-threatening DR.
Revolutionizing eye care for diabetic patients
In collaboration with the VGH Diabetes Centre, Karst and VCHRI scientist Dr. David Maberley will employ a cutting-edge, non-invasive diagnostic test called optical coherence tomography angiography (OCTA) to evaluate patients who are at increased risk of DR worsening because of poor blood sugar control.
OCTA uses a laser light to take high-resolution images of the tiny blood vessels of the eye’s retina. The scans reveal the vascular networks in the eye, as well as the thickness of patients’ retinas. Researchers will evaluate scans of patients’ eyes over a 12-month period, and will later use this data to better understand how DR develops and can worsen.
Unlike previous diagnostic tools that used to involve intravenous dye injections and exposure to a fairly bright light for 10-20 minutes, an OCTA scan can be completed within a few seconds and does not require the use of an intravenous dye.

“There are considerable challenges in providing regular retinopathy screening to all people living with diabetes in Canada,” says Maberley. “A new approach that can predict who will or will not develop vision loss will ultimately prevent visual disability and blindness.”
The results of Karst and Maberley’s study could establish a framework for using OCTA to diagnose patients at high-risk of developing sight-threatening DR. With this greater understanding of the disease, patients at low-risk of developing severe DR could be spared frequent and time-consuming eye exams, freeing up clinical time for patients who are at a higher risk of developing sight-threatening complications.
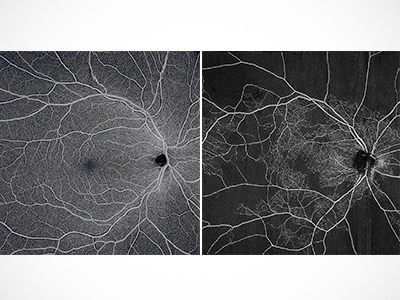
“Early detection of DR can save eyesight,” says Karst. “This research will help us better understand how the disease progresses over time so that we can prevent it from doing long-lasting damage to patients’ eyesight and quality of life.”
The clinical trial is recruiting participants between the ages of 18 and 90. For more details and to learn if you qualify, contact Sonja Karst at 604-875-5475 sonja.karst@ubc.ca or Theresa Wiens at 604-875-4111 ext. 62544 twiens@eyecarecentre.org.
Funding for this study is provided by Diabetes Action Canada, a Strategic Patient Oriented Research (SPOR) Network in Chronic Disease that is part of the Canadian Institutes of Health Research (CIHR).


